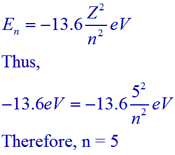If the total energy of an electron in a hydrogen atom in an exicted state is -3.4 eV , then - YouTube

derivation of: energy of an electron in the nth orbit of Hydrogen atom - Physics - Atoms - 12867151 | Meritnation.com
energy of electron in orbit of H atom is 1.51eV. Wavelength produced by the electron in same orbit if 1st orbit of H have radius x i
a) Using Bohr's postulates, obtain the expression for total energy of the electron in the nth orbit of hydrogen atom. - Sarthaks eConnect | Largest Online Education Community

Using rutherford model of atom derive an expression for the total energy of the electron in hydrogen - Brainly.in
Derive the energy expression for hydrogen atom using Bohr atom model. - Sarthaks eConnect | Largest Online Education Community
Assuming the expression for radius of the orbit, derive an expression for total energy of an electron in hydrogen atom. - Sarthaks eConnect | Largest Online Education Community

SOLVED:Calculate the energy, in joules, of a hydrogen atom when the electron is in the sixth energy level.